Tailor learning experiences to your organization
Help drive productivity and compliance with comprehensive learning management. Leverage a tailored training experience and align content creation, an expert library, distribution and reporting all in one platform.
Hit the ground running with the ULTRUS Learning course catalog
The ULTRUS Learning course catalog is accurate, engaging and informative e-learning and regulatory training. Our courses cover best practices in leadership, safety and compliance. With coverage of over 1000 courses maintained by UL Solutions experts, trust that you are enabling your organization with the latest best practices.
Comprehensive library
Tailor content to your industry and role, with wide coverage including life sciences, manufacturing, construction and logistics.
PureSafety® EHS catalog
Bring workplace safety to the forefront of your organization with one of seven OSHA-authorized online training providers for general industry and construction.
Life sciences
Utilize the same content used and co-developed by the U.S. FDA to drive compliance and manage qualification.
Create and customize e-learning across the organization
Easy-to-use, collaborative tools are needed to empower your company’s subject matter experts to produce professional employee learning and process training courseware. ULTRUS Learning offers an easy way for your organization to build course content without a strong instructional design background. Augment our off-the-shelf content library or create effective and interactive courses that you can easily share through ULTRUS Learning or your preferred learning management system.
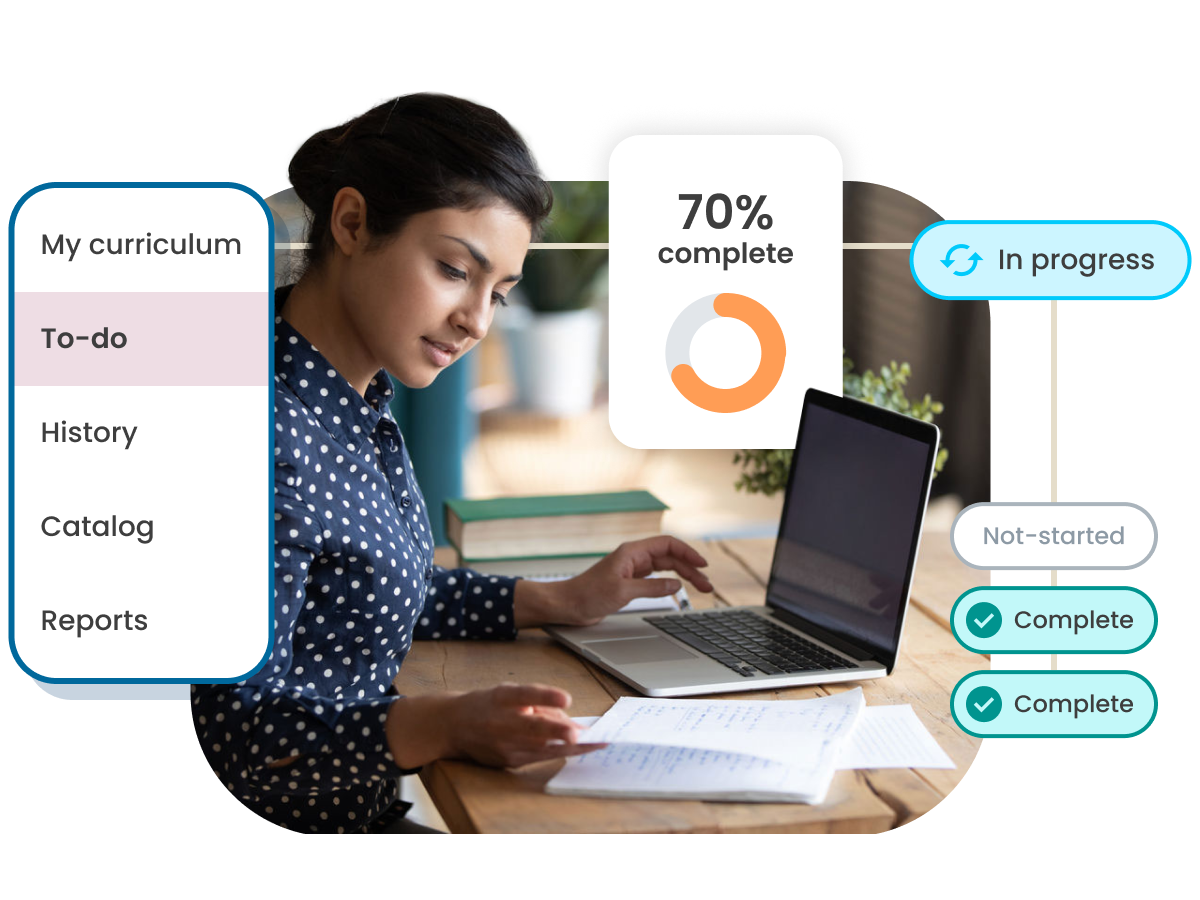
Roll out an adaptive, targeted and engaging learning culture
Make the most out of your employees’ training time by driving tailored experiences that focus on the main areas they need to grow. Centralize all your training in one place for complete oversight on employee growth. Leverage AI to automatically transcribe and translate content to closed captions and scale globally.
LearnShare™ LMS
See how ULTRUS Learning can bring knowledge to the forefront, decentralize learning and build a scalable learning culture.
Tailored learning
Leverage advanced audience mapping to deliver tailored learning experiences based on persona attributes, prior courses, survey answers or xAPI statements.

Employee onboarding
Automate and customize employee onboarding to reduce employee time to value.
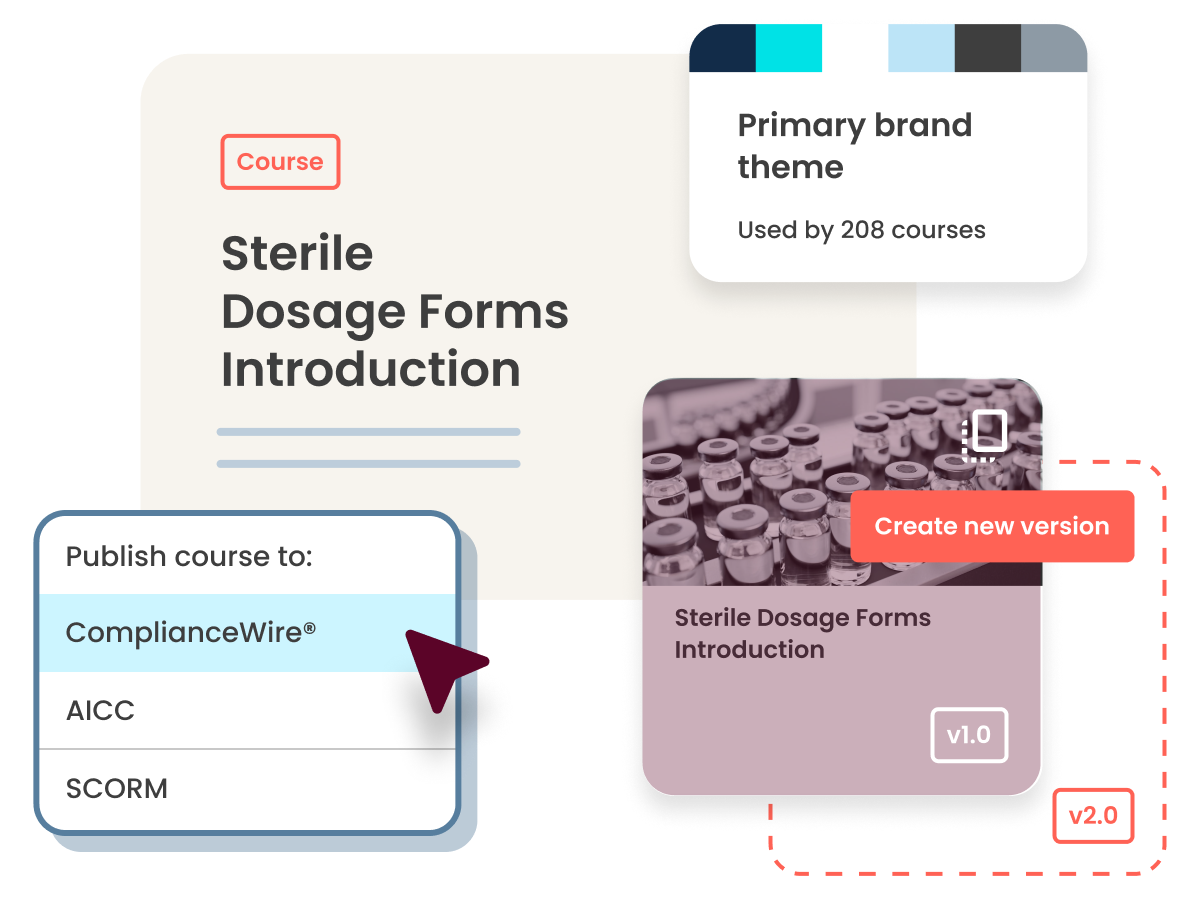
Meet FDA and regulatory requirements with ComplianceWire® LMS
An industry-leading learning management option for compliance and qualification management created for life sciences organizations, ComplianceWire Learning and Qualification Management System is an isolated instance of ULTRUS Learning that natively maintains compliance with U.S. Food and Drug Administration (FDA) 21 CFR 11 and EU Annex 11 electronic signature and records requirements.


ComplianceWire® LMS
Support the safety and effectiveness of products through robust, automated training capabilities for employees.
Fully auditable reporting
Track employee qualifications by role, product or department and easily respond to audits with out-of-the-box reporting.
Supplier qualifications
Manage supplier communications, qualifications and training to support GxP excellence across your supply chain.