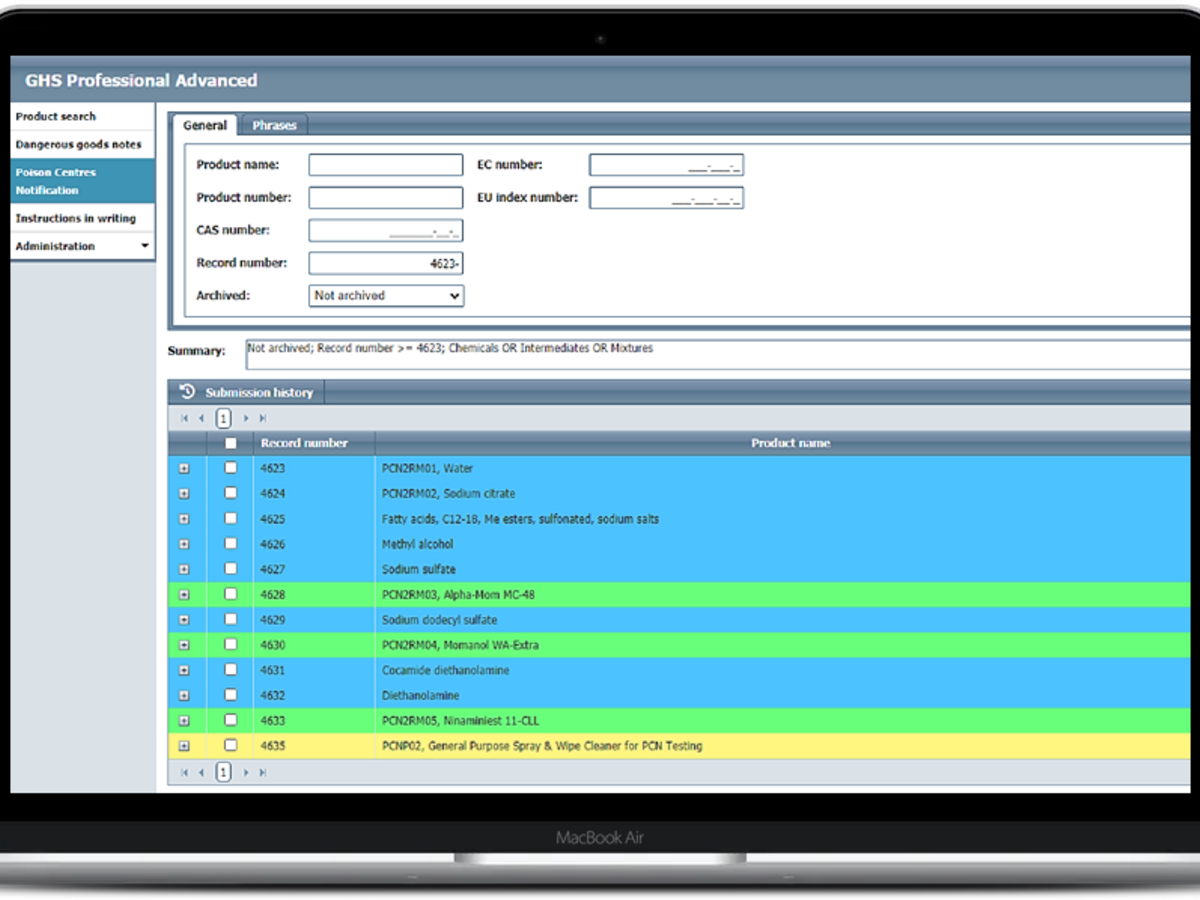
Simplified creation of compliant Poison Centre Notifications
UL Solutions’ team of Poison Centre experts have worked closely with ECHA to develop a comprehensive Poison Centre Notification solution to help you meet your current and upcoming PCN obligations quickly and effectively.
Our brand new PCN module integrates seamlessly with GHS Professional and provides a comprehensive solution for making Poison Centre Notifications required under Article 45 of CLP.
- Leverages familiar and powerful GHS Professional concepts and tools
- Generates and stores PCNs for your products
- Automatically pulls relevant data from the EU SDS
- Prompts users to add information that is legally required but not present on the SDS (e.g. packaging information)
- Define and manage your legal entities and their VATs or suppress VAT use
- Allows manipulation of the XML within a user-friendly, readable interface
- Takes advantage of translations in the GHS Professional database for language submission needs
- UFI number can be generated within the system or manually input
- Smart interface which allows users to track submissions and review the submission history
- Submissions are checked against ECHA-defined validation rules, and will prompt users where there are failures or advisories
- Supports identification and reporting of generics (perfumes, colorants)
- Submits electronically via ECHA’s system-to-system connectivity
Complete the contact form to get in touch with our experts.
Get connected with our sales team
Thanks for your interest in our products and services. Let's collect some information so we can connect you with the right person.
Submitting data to Poison Centres can be a complex process.
Companies selling products into the EU needs to comply with current and upcoming Poison Centre regulations.
UL Solutions’ new PCN Module dramatically reduces the amount of manual work required to notify poison centres throughout Europe. The module will:
- Automatically take relevant data from your safety data sheets (SDS)
- Allow you to fill in any gaps that ECHA requires for the submission once the SDS data has been transposed
- Allow you to validate the entry before submission
- Send the submission to ECHA and the Member States automatically via the module
The PCN module provides an automated, optimized end-to-end solution for the generation and submission of poison centre notifications, and will enable you to meet your obligations quickly and effectively.
Poison Centres: What You Need To Know
For more information about Poison Centres, check out our dedicated Poison Centres Homepage, where you can find the latest resources, including White Papers, FAQs and Expert Articles to help you understand your obligations under Annex VIII. You can also learn more about our expert tools and services to help you meet your compliance requirements.