
The expanding scope of medical device testing
Medical product safety, performance and quality paradigms are shifting as devices become more interoperable, hospital environments become more complex and digital health technologies proliferate. Only through third-party testing and certification can manufacturers demonstrate claims of safety, quality and performance for their products, even as they innovate.
Leverage our rigorous testing and certification capabilities

Demonstrate device safety and performance
Meet regulatory requirements and industry standards to deliver robust devices.

Meet market access requirements
Use testing and certification results to address regulatory requirements.
Demonstrate electromagnetic compatibility (EMC)
Meet EMC regulations and mitigate risks of electromagnetic interference.
How we help customers deliver safe and compliant products

Medical Device Performance and Safety Testing
We conduct independent, rigorous testing and certification of medical devices to help manufacturers demonstrate safety and performance claims according to regulatory requirements and industry standards.

Quality Management System Audit Service
We provide independent assessment of health and life sciences companies’ QMSs to verify compliance to the ISO 13485 standard for medical device quality management systems. ISO 13485 certification is required by regulators in many markets.

SaMD and Health Software Certification
We offer testing and certification for software as a medical device (SaMD) according to industry standards including IEC 82304-1, IEC 60601-1 part 14 and IEC 62304, which can help demonstrate SaMD safety and quality.

Medical Device EMC Testing and Certification
In environments where electromagnetic interference poses risks to patients, we provide electromagnetic compatibility (EMC) testing and certification to help demonstrate conformance to the IEC 60601-1-2 standard for safety and essential performance.

Medical Device Packaging Testing
We offer medical device packaging validation and stability testing according to standards such as ISO 11607-2:2019 for packaging terminally sterilized medical devices. Our testing covers seal integrity, sterility, qualification of packaging materials, air permeability and more.

Restricted Substances Testing and Advisory Services
Our testing and advisory services help customers demonstrate compliance to requirements such as the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) and Restriction of Hazardous Substances (RoHS) regulations.
Learn more
Meet our experts
“Together, we create solutions that help Medtech innovators ensure essential technologies reach the market safely and effectively.”
Pamela K. Gwynn
Principal Engineer, Consumer, Medical and Information Technology (CMIT)

Featured resource
U.S. Market Access for Medical Devices: FDA and ASCA Program 2024
Our on-demand webinar covers the U.S. FDA’s Accreditation Scheme for Conformity Assessment (ASCA) program to boost confidence in device testing.
Related testing and certification resources
Related Health and Life Sciences market access offerings

Product and Process Development
UL Solutions offers human factors engineering advisory and software support to help product developers and R&D professionals lacking internal resources and expertise to develop safer, more effective and more usable medical products*.
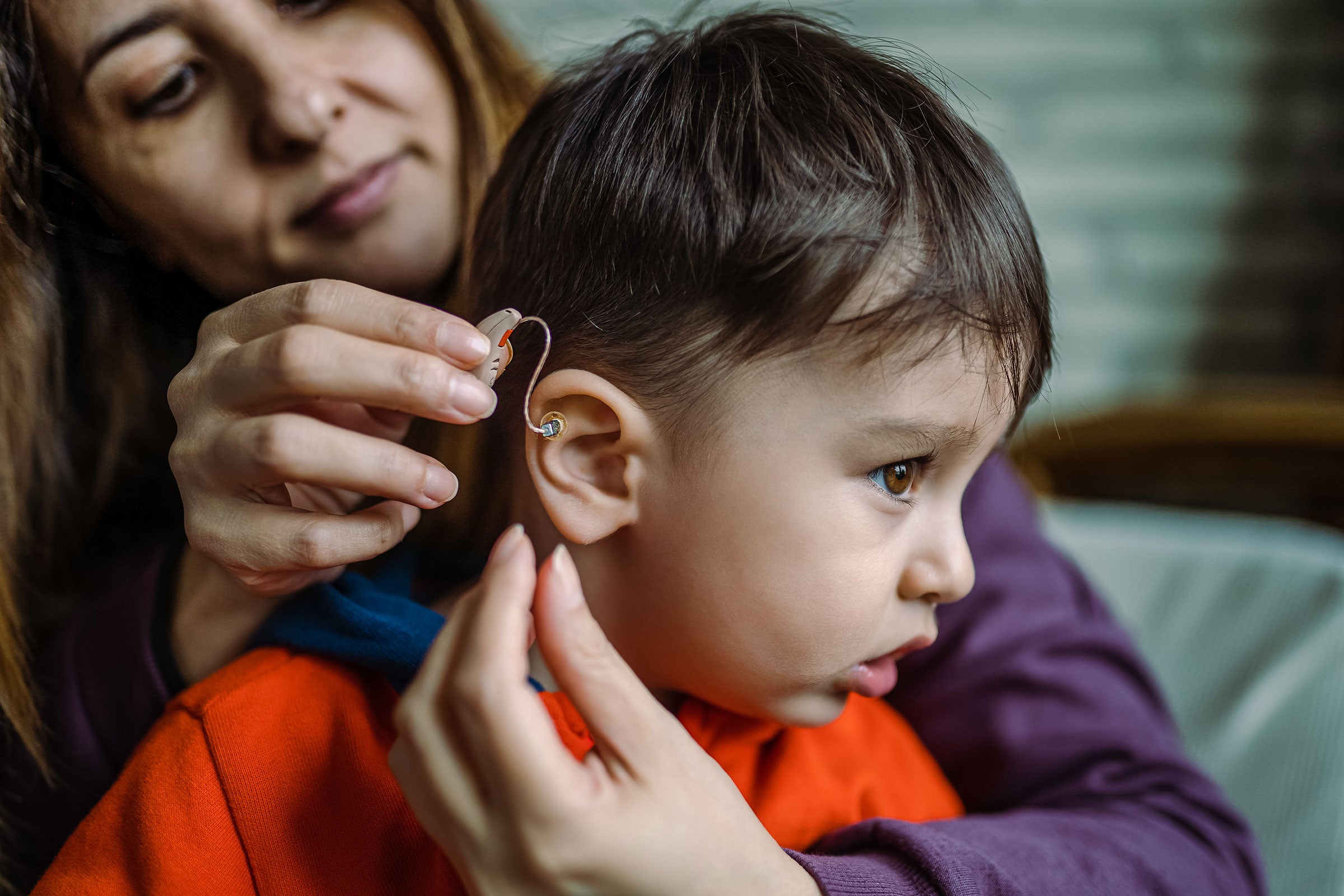
Regulatory Readiness and Compliance
Our network of regulatory experts helps customers achieve market access in more than 20 countries. We provide knowledge and tools to automate regulatory affairs processes to streamline market submissions and keep up with regulatory changes*.
Learn more
Learn more about our Health and Life Sciences offerings
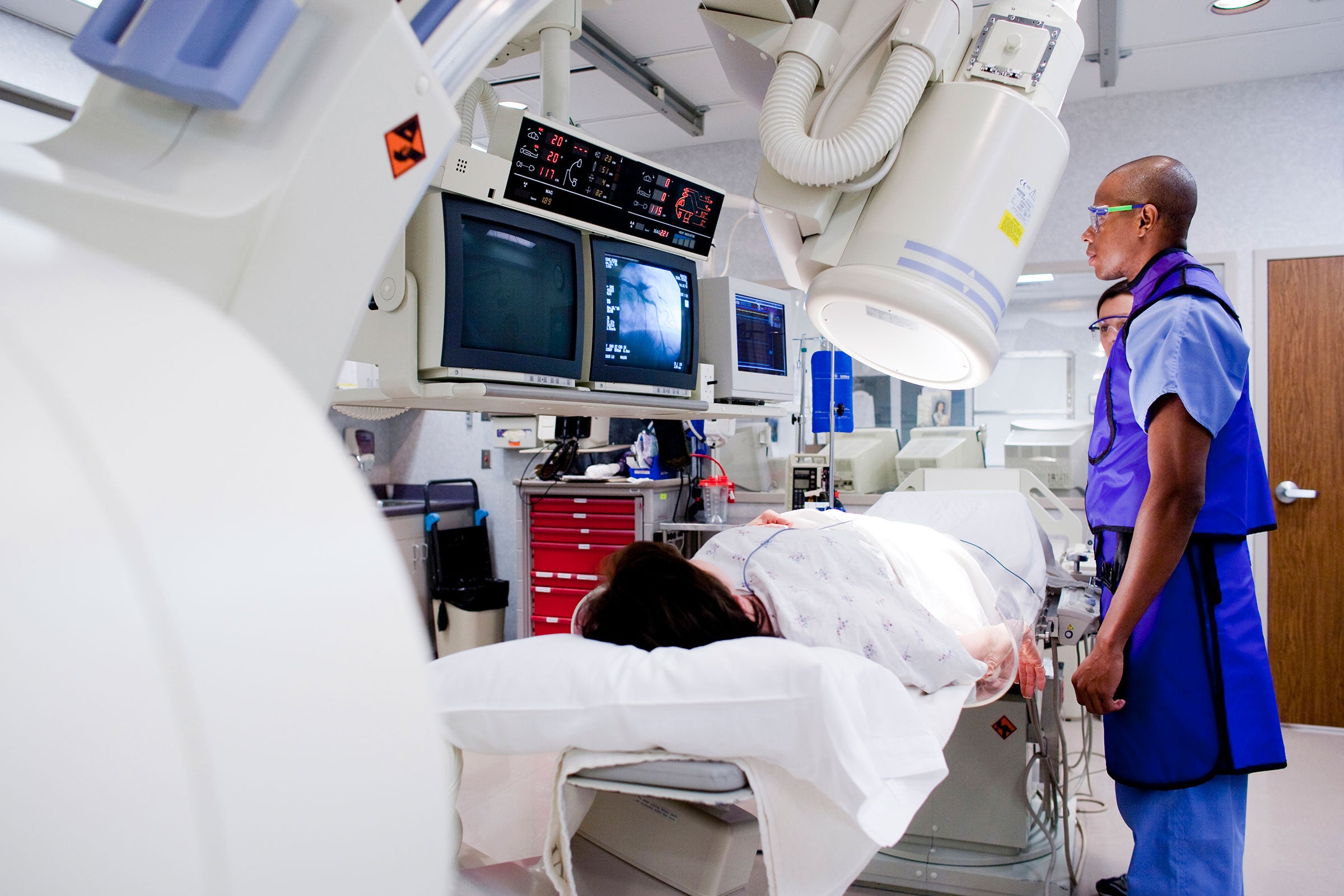
Market Access
UL Solutions provides software and expert consulting services to support product development and global regulatory compliance. Our testing and certification help customers demonstrate safety and performance.
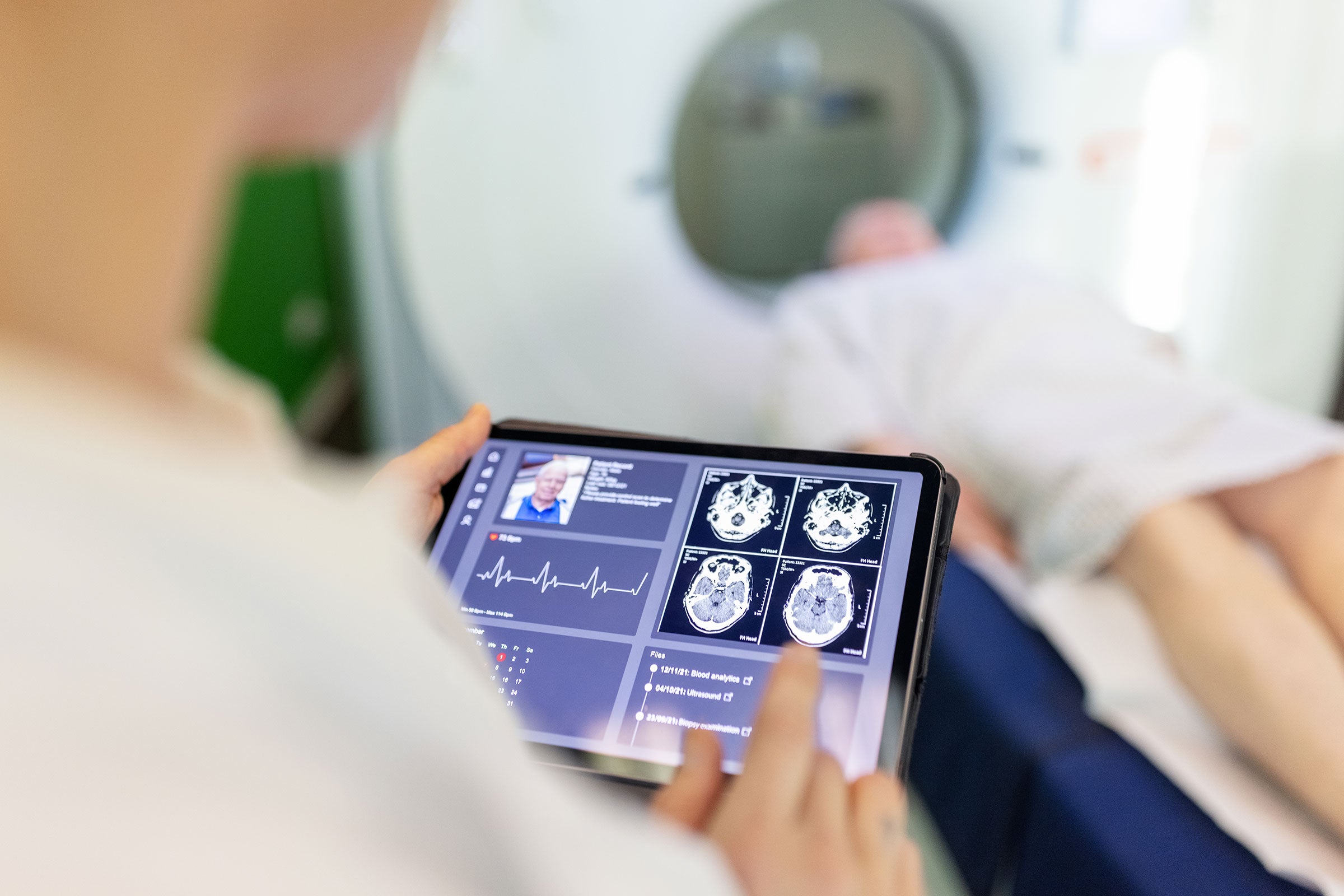
Cybersecurity
We offer software, testing and expertise to manage cybersecurity risks and develop safer manufacturing processes under the Industrial Internet of Things (IIoT).

Workforce Training and Competence
We offer learning management software to help companies implement training programs, standardize manufacturing processes and keep pace with regulatory changes.

Supply Chain Resilience
Our software and services provide visibility into firms’ supply chain networks, helping to minimize disruptions and mitigate supplier- and product-related risks.

ESG Stewardship
We offer software and advisory expertise to manage ESG reporting, reduce carbon emissions and more sustainably bring medical products to market.
Learn more
* Within UL Solutions, we provide a broad portfolio of offerings to many industries. This includes certification, testing, inspection, assessment, verification and consulting services. In order to protect and prevent any conflict of interest, perception of conflict of interest and protection of both our brand and our customers’ brands, UL Solutions has processes in place to identify and manage any potential conflicts of interest and maintain the impartiality of our conformity assessment services.
Validate safety and performance claims
Leverage our testing and certification.







