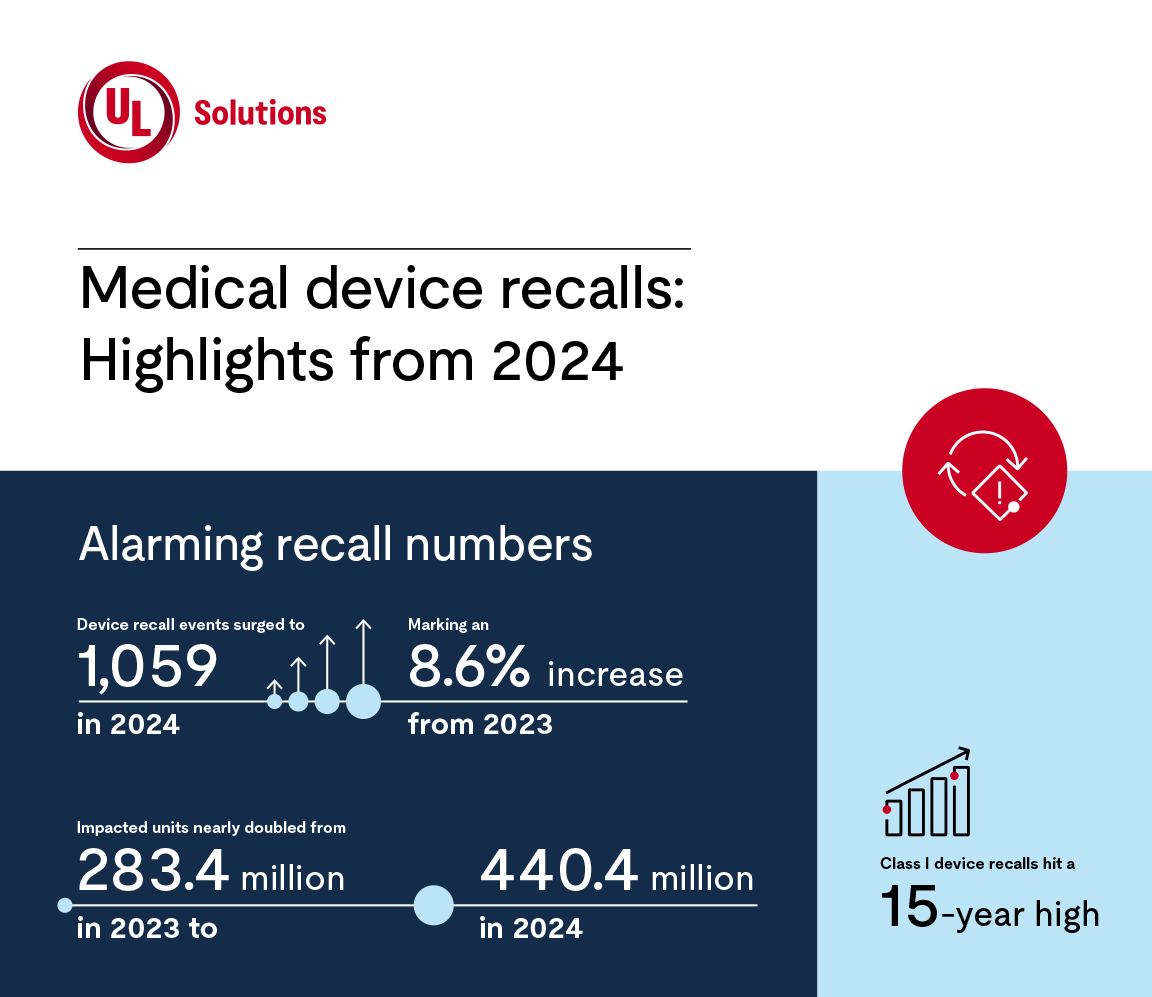
Medical device recalls in the U.S. market are on the rise. Over the past three years, recalls of lower-risk Class I devices have surged from 7.7% of all events in 2023 to 10.8% in 2024.
The U.S. Food and Drug Administration’s (FDA’s) Center for Devices and Radiological Health (CDRH) is launching a pilot program to increase transparency and improve recall communication with patients and healthcare providers. The program provides early alerts for potentially dangerous device failures, updates and corrections, and applies to cardiovascular, gastrorenal, general hospital, obstetrics and gynecological, and urological devices. Event thresholds for the FDA’s enhanced public alert system have yet to be established.
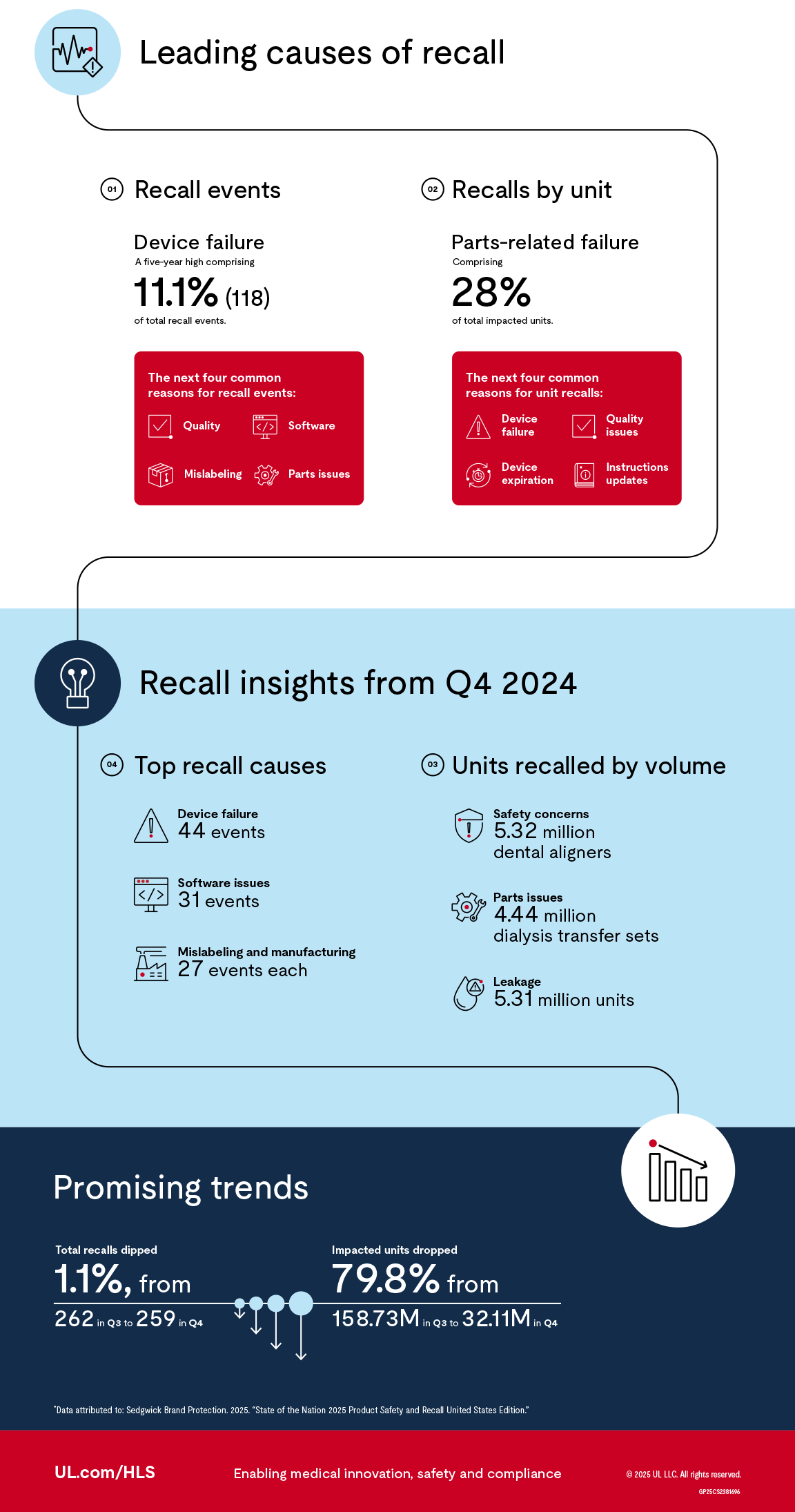
With total medical device recall events increasing and impacted units nearly doubling since last year, staying informed is essential. Here’s a closer look at 2024’s key recall data.

Download our infographic
Medical device recalls: Highlights from 2024
Related resources
Get connected with our sales team
Thanks for your interest in our products and services. Let's collect some information so we can connect you with the right person.





